

India and many countries around the world have set up NPP to make recently approved and advanced medicines available to the patients who have no other treatment options available to them. Under NPP, patients with serious or life-threatening diseases can get access to lifesaving drugs that are not approved in their home country. NPP helps patients in India to experience the clinical benefits of new and latest medicines approved by the US FDA, European Medical Agency, etc. Many of the medicines procured under Named Patient Program or NPP are Lifesaving in nature. However, there are many more therapeutic options that can help in prolonging survival with improved quality of life.
DigePharma Global focuses on the sourcing and supply of advanced medicines for serious or life-threatening diseases under NPP. We facilitate the process of obtaining the medicines from start to finish and make the lifesaving medicines available to the patients quickly and safely. Our experienced team handles the regulatory and logistical processes involved in the approval for the import of the medicines, sourcing, and delivery of the medicines to the patients as per the Good Distribution Practices (GDP) prescribed by WHO. DigePharma Global uses temperature-controlled packaging as per the product storage requirements that ensures proper storage conditions from origin to end user. Temperature is monitored en-route by adding temperature data loggers to the shipment.
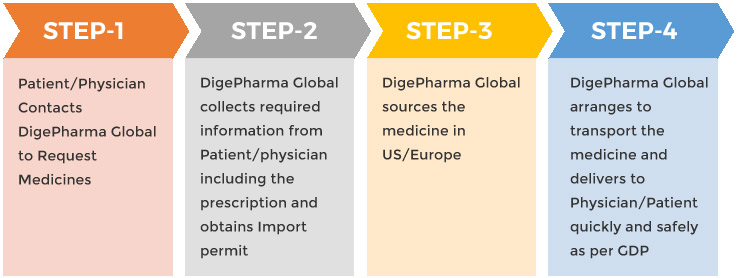
With Named Patient program, the patient and his/her physician discuss and decide if the medicine available in US/Europe is to be procured and used for the treatment. DigePharma Global works with physicians and their patients and assists them in getting the necessary import permit from the Government of India, procuring the medicines from US /Europe and transporting them safely to the physicians/patients.